Rat Granulocyte Macrophage Colony Stimulating Factor Recombinant
Categories: HematopoietinsIL-3 familyRecombinant Rat Cytokines$70.00 – $2,900.00
Description
Accession
P48750
Source
Optimized DNA sequence encoding Rat Granulocyte Macrophage Colony Stimulating Factor mature chain was expressed in Escherichia Coli.
Molecular weight
Rat Granulocyte Macrophage Colony Stimulating Factoris generated by the proteolytic removal of the signal peptide and propeptide,this molecule has a calculated mass ofkDa. Recombinant RatGM-CSFis a monomer protein, consisting of amino acidsandmigrates as an approximately14 kDa protein under reducing and non reducing conditions on PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED50 was determined by the dose-dependent stimulation of the proliferation of Murine FDC-P1 cells is <.1 ng/ml, corresponding to a specific activity of > x units/mg.
Protein Sequence
MWLQNLLFLG IVVYSFSAPT RSPNPVTRPW KHVDAIKEAL SLLNDMRALE NEKNEDVDII SNEFSIQRPT CVQTRLKLYK QGLRGNLTKL NGALTMIASH YQTNCPPTPE TDCEIEVTTF EDFIKNLKGF LFDIPFDCWK PVQK
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant rat GM-CSF was lyophilized from a 0.2 μm filtered PBS solution.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Molecular function
Molecular function
Methods
Cells and antibodies
- Human monocytes were obtained from leukocyte-enriched buffy coats obtained from healthy volunteer blood donors drawn at the of in accordance with the written approval of the Director of the and the and Ethics Committee of the University of, Medical and Health Science .
- Written informed consent was obtained from the donors prior to blood donation, and their data were processed and stored according to the principles expressed in the Declaration of Helsinki.
- Human monocytes were isolated as described previously
6-OHDA treatment and cytokine injection
- Animals were kept under pentobarbital sodium anesthesia (50 mg/kg) and placed in a stereotactic instrument .
- 6-OHDA was dissolved in saline containing ascorbic acid (10 μg/μL dissolved in 1% ascorbate-saline), kept on ice (4°C) and protected from light to minimize oxidation, until use.
- The rats were then given uni- or bilateral injections of 6-OHDA.
- Unilateral injection was employed for immunohistochemical analyses, and bilateral injection was used for all other studies.
- For unilateral injection, 5 μL of 6-OHDA was drawn into a Hamilton syringe and then injected into the right side of the striatum, through a hole made on the skull at 1 mm anterior to bregma and 3 mm lateral from the midline.
- The depth of the needle tip was 5 mm from the skull surface.
- The same amount of 6-OHDA was injected into the left side of the striatum for bilateral injection.
- The rate of fluid injection was 1 μL/min.
- The needle was left…
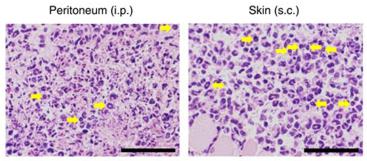
γIMCs are the source of IFN-γ in severe invasive GAS infections.
- (f) Sorted IMCs were cultured with control medium (Med), G-CSF (50 ng ml−1), M-CSF (10 ng ml−1), GM-CSF (10 ng ml−1), or IL-5 (10 ng ml−1) for 2–4 days, and their absolute numbers were counted on the indicated days.
Cell Isolation and Culture
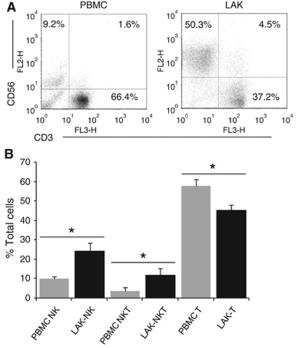
- Peripheral blood mononuclear cells (PBMCs) were separated by centrifugation through Ficoll–Hypaque .
- CD14+ monocytes were isolated by positive selection (CD14 beads , , ) and immature moDCs were generated by culturing the isolated monocytes for 5 days in complete medium (RPMI 1640, supplemented with 10% of heat-inactivated fetal calf-serum, , ) with 500 U/ml rhIL-4 and 1000 U/ml GM-CSF .
- One-third of complete medium, including cytokines, was replaced every 3 days.
- For some experiments primary mDCs were purified from circulating elutriated monocyte fraction on a MoFlo XDP Cell Sorter .
- Cells were stained in PBS containing 2% FBS using fluorochrome-conjugated antibodies and mDCs were defined as CD14−HLA-DR+CD11c+ with purity >98%
DC Preparation and in vitro Challenge
- Enriched DCs were prepared by depleting T and B cells from total splenocytes using purified rat anti-mouse CD3 and anti-mouse CD19 mAbs, then anti-rat IgG magnetic beads , followed by further enrichment using CD11c+ magnetic beads (CD11c+ isolation kit ).
- The enriched CD11c+ DCs were seeded in a 96-well round-bottom tissue culture plate (5×105 cells/well) in RPMI-1640 supplemented with 10% FCS, L-glutamine, Na pyruvate, Hepes, non-essential amino acids and 10−5 M β-ME with GM-CSF (10 ng/ml, ) and challenged with the TLR9 agonist, unmethylated CpG ODN 1668 (CpG; 6 µg/ml).
- The effect of injury on maturation of cDCs was examined at 24 h post-TLR9 activation and pDCs at 40 h post-activation.
- After activation, cells were washed and stained with APC-conjugated anti-mouse CD11c, PE Texas Red-conjugated anti-mouse B220, FITC-conjugated anti-mouse PDCA, PE-conjugated anti-mouse CD80, or CD86 and biotin-conjugated MHC Class-II with…
Generation of bone marrow-derived DCs and PrA treatment
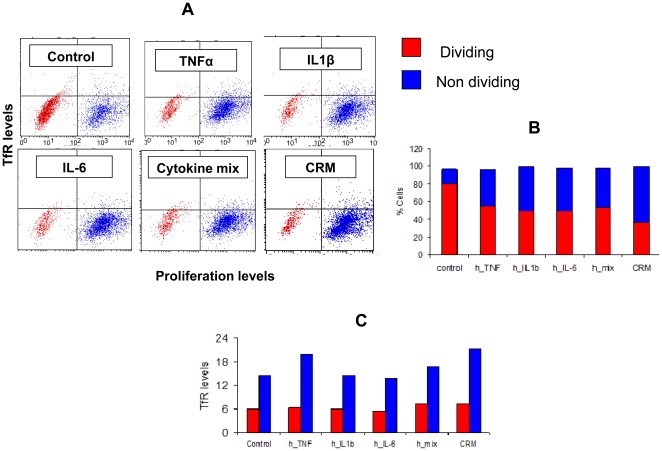
- DCs were derived from the bone marrow precursors of Wistar rats as described previously 6 cells/ml in RPMI 1640 medium supplemented with 10 ng/ml each of rat GM-CSF and IL-4 .
- To induce DC maturation, cells at day 6 in vitro were treated for 48 h with lipopolysaccharide (LPS, 10 ng/ml, , ).
- The DC phenotype was confirmed by anti-OX62 labeling and flow cytometry using a FACS Calibur system (detailed below).
- Only those preparations with a purity >85% DCs were used for subsequent experiments.
- In other experiments, the DCs at day 6 were treated for 48 h with LPS plus different doses of PrA.
Human macrophage differentiation assay
- CD14+ monocytes were cultured in complete medium'>RPMI-Glutamax™ medium containing GM-CSF alone (10 ng/ml) from day 0 to day 3 and GM-CSF (2 ng/ml) plus CSF-1 (10 ng/ml) from day 3 to day 6.
- Antibodies, F(ab’)2 or the CD115 TK inhibitor GW2580 (LC Labs) were added at day 0 and day 3.
- At day 6, cells were counted (5 microscope fields /well), harvested and pools of triplicate wells were analyzed by FC using antibodies from .
- For staining surface FcγR, anti-CD16 clone 3G8, anti-CD32 clone 3D3 (recognizing both FcγRIIa and FcγRIIb,1.
- Secreted cytokines were titrated by multiplex .
2.5. Primary splenocyte and bone marrow-derived dendritic cell (BMDC) culture
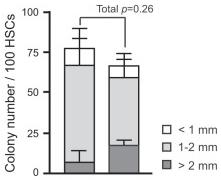
- Immature BMDCs were isolated using a protocol modified from previous publications 6 cells) were then cultured in 1 ml complete medium for 7 d in the presence of recombinant rat IL-4 (10 ng/ml, , , ) and recombinant rat granulocyte-macrophage colony-stimulating factor (GM-CSF, 10 ng/ml, , , ).
- At day 7, immature BMDCs were resuspended in complete medium for maturation.
- Mature BMDCs (1×106 cells/well) were incubated with LPS (1 µg/ml, from
E. - coli, , , ) for 12 h and then treated with culture supernatant of
F. - prausnitzii (1 µl), sodium butyrate solution (0.005834 µmol) and denatured
F. - prausnitzii or
longum bacteria (1×106 CFU/well, BMDCs: bacteria = 1:1) at 37°C for another 12 h. PBS treated BMDCs were used as a control. - Each group treatment was repeated four times.
- The supernatant was collected and stored at −20°C.
Culture and characterization of DCs
- DCs were generated from rat bone marrow as previously described[6 cells/mL in medium'>RPMI-1640 medium containing 10% fetal bovine serum , 2 mM glutamine, 100 μg/mL penicillin , 100 μg/mL streptomycin , 500 U/mL of recombinant rat granulocyte-macrophage colony- stimulating factor , and 500 U/mL of recombinant rat interleukin-4 .
- On days 2 and 4, half of the culture supernatant was removed and replaced with fresh medium containing recombinant rat granulocyte macrophage colony- stimulating factor and interleukin-4.
- On day 6, all the loosely adherent cells were harvested, centrifuged and counted.
- Then C6 glioma cell lysates and DCs (a ratio of 3 tumor cells to 1 DC) were incubated in fresh medium with recombinant rat granulocyte macrophage colony-stimulating factor and interleukin-4 together at 37°C in 5% CO2.
- To stimulate DC maturation
in vitro , on day 8, the cultures were additionally…