mouse Thrombopoietin Recombinant
Categories: HematopoietinsRecombinant Mouse CytokinesSingle chain family$70.00 – $3,900.00
Description
Accession
P40226
Source
Optimized DNA sequence encoding mouse TPO erythropoeitin like domain was expressed in Escherichia Coli.
Molecular weight
Native mouse TPO, generated by the proteolytic removal of the signal peptide and propeptide,The molecule has a calculated molecular mass of approximately 19 kDa. Recombinant mouse TPO is a monomer protein consisting of 174 amino acid residue subunits, and migrates as an approximately 19 kDa protein under reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC.
Biological Activity
The ED(50) was determined by the dose-dependent proliferation of human MO7e cells was found to be less than.0 ng/ml.
Protein Sequence
MELTDLLLAA MLLAVARLTL SSPVAPACDP RLLNKLLRDS HLLHSRLSQC PDVDPLSIPV LLPAVDFSLG EWKTQTEQSK AQDILGAVSL LLEGVMAARG QLEPSCLSSL LGQLSGQVRL LLGALQGLLG TQLPLQGRTT AHKDPNALFL SLQQLLRGKV RFLLLVEGPT LCVRRTLPTT AVPSSTSQLL TLNKF PNRTS GLLETNFSVT ARTAGPGLLS RLQGFRVKIT PGQLNQTSRS PVQISGYLNR THGPVNGTHG LFAGTSLQTL EASDISPGAF NKGSLAFNLQ GGLPPSPSLA PDGHTPFPPS PALPTTHGSP PQLHPLFPDP STTMPNSTAP HPVTMYPHPR NLSQET
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant mouse TPO was lyophilized from a 0.2 μm filtered PBS solution.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Molecular function
Molecular function
Methods
Expansion of CD34+ cells in serum free medium
- Isolated CD34+ cells were seeded at a density of 5×104cells per 500 µl of medium'>Stempro medium , in 24-well tissue culture plates .
- Growth factors used were IL-6, SCF, TPO, and Flt-3-L at a final concentration of 25 ng/ml with (test cells) and without (control cells) the addition of either zVADfmk −100 nM or zLLYfmk −10 µM (MP, , ).
- After the 10th day of culture, the cells were collected from the suspension culture, and centrifuged (1000 rpm for 5 minutes).
- The cells were used for assessing the
in vitro homing properties or were used forin vivo homing studies in NOD/SCID mice.
Lentiviral transduction of bone marrow cells
- Freshly isolated bone marrow (BM) cells were plated in Iscove's Modified Dulbecco's Medium (IMDM) (31980, , ) containing 5% fetal bovine serum (FBS), 1% penicillin/streptomycin, 200 mM glutamine, 1% non-essential amino acids, 1% sodium pyruvate, 50 μM 2-mercaptoethanol, stem cell factor (SCF, 10 ng/ml, , ), Flt3-L (10 ng/ml), IL-11 (10 ng/ml), thrombopoietin (TPO, 10 ng/ml), IL-6 (10 ng/ml), and IL-3 (10 ng/ml).
- Bone marrow cells were spin-infected with 8 μg/ml polyprene and lentivirus (MOI of 0.1–0.5), at 2,500 rpm for 90 min.
- BM cells were then incubated for 3 hours at 37°C, counted and injected into NOD.SCID mice.
Generation and isolation of MLL-AF9 leukemia cells
- MLL-AF9 mouse leukemias were generated in Dr. David Scadden's laboratory as described below.
- Actin-DsRED mice (JAX) were backcrossed for 10 generations onto C57BL/6J mice (JAX).
- These mice were sacrificed four days after injection with 150 mg/kg 5FU.
- BM cells were isolated from femurs and tibias, and red blood cells were lysed using ACK lysing buffer .
- Cells were incubated in RPMI supplemented with 20% FBS, 1% Pen-Strep, 6 ng/ml of IL-3 , 10 ng/ml of TPO , 10 ng/ml of IL-6 , at 37°C 5% CO2 overnight.
- MLL-AF9 was introduced by spin-infection (1 000 g for 90 minutes) using a retroviral vector (MSCV-MLL-AF9-neo) 6 live cells per mouse were injected into lethally irradiated (9Gy) C57BL/6 recipient mice 12 hours after viral infection.
- The mice were sacrificed once they became moribund.
- BM cells were isolated as described above, and subjected to ACK lysis.
- 200 000 cells were injected into sublethally irradiated (4.5 Gy)…
Single Cell Hematopoietic Colony Forming in vitro Assay
- celltype'>BM-derived celltype'>cells were isolated, stained as described above using combination of CD45-FITC, Lin-PE, Sca-1-PE-Cy5, CD105-PE-Cy7 or c-Kit-APC-Cy7 antibodies, and sorted with MoFlo XPD cell sorter.
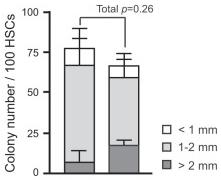
- Only DAPI-negative cells with integral membrane were chosen, using gating strategy presented in 2), then wells with colonies were counted, the colonies were harvested, diluted with PBS to final volume of 250 µl, cytospined (1,000 rpm, 10 minutes, room temperature), air-dried, and stained using’s method with Hemacolor Kit .
In vitro myeloid and erythroid cell differentiation assays
-
Semi-solid cultures of freshly isolated, unfractionated BM or spleen cells and sorted progenitor populations from uninfected or
P. - chabaudi-infected mice were prepared using Methocult M3234 ( Cell ) supplemented with SCF (50 ng/ml), Flt3L (5 ng/ml), GM-CSF (1 ng/ml), M-CSF (100 ng/ml), TPO (10 ng/ml) (all) for myeloid differentiation.
- Colonies were assessed and counted under an inverted microscope from day 3 to day 12.
- For confirmation of colony types, colonies were picked with fine-drawn Pasteur pipettes, spun down on slides, and after staining with Giemsa evaluated by light microscopy.
Bone marrow transduction
- Retroviral transduction of bone marrow was done as previously described (6/ml) were prestimulated for 48 hours in IDMEM containing 10% Premium FBS , 2% Penicillin/Streptomycin, and 100 ng/ml each of recombinant murine TPO, G-CSF, and SCF .
- For primary transduction, cells were resuspended in appropriate retroviral supernatant containing growth factors and seeded onto fibronectin coated wells ((
Murine Marrow Culture, Transduction, FACS Analysis, and Transplantation
- Marrow isolated from 8–16 week old C57BL/6 female mice was subjected to red cell lysis with NH4Cl, and the cells were lineage-depleted using biotin-conjugated B220, Gr-1, Mac-1, Ter119, and CD3 mouse Lineage Cocktail , anti-biotin microbeads, and MACS columns .
- The cells were then cultured in Iscove’s medium'>modified medium'>Dulbecco medium (IMDM) with 10% heat-inactivated fetal bovine serum (HI-FBS) containing 50 ng/mL murine stem cell factor (SCF), 100 ng/mL murine FLT3 ligand (FL), and 10 ng/mL murine thrombopoietin (TPO) for 24 hrs.
- Lentiviral particles were generated by transient transfection of 293T cells with plasmids at a ratio of 3.75 µg pLKO.1 vector: 5 µg pCMV-ΔR8.91: 1.25 µg pMD.G(VSV.G) per 100 mm dish using 20 µL Lipofectamine 2000 .
- Lentiviral supernatants collected at 48 hr were concentrated with AmiconUltra filters .
- Retroviral particles were packaged similarly at a ratio of 8 µg MIG or pBabe vector: 2…
Colony-forming unit assays
- Mouse BM cells were harvested via flushing of the long bones with medium'>Dulbecco medium'>modified medium'>Eagle medium (DMEM;, , , ) supplemented with 10% fetal bovine serum (FBS, , , ), was followed by filtering through a 70-µm nylon mesh cell strainer, to remove bone debris.
- BM mononuclear cells were cultured in medium'>MethoCult medium'>M3231 medium supplemented with 50 ng/ml Thrombopoietin (TPO, , ), for 7 days according to the manufacturer's protocols .
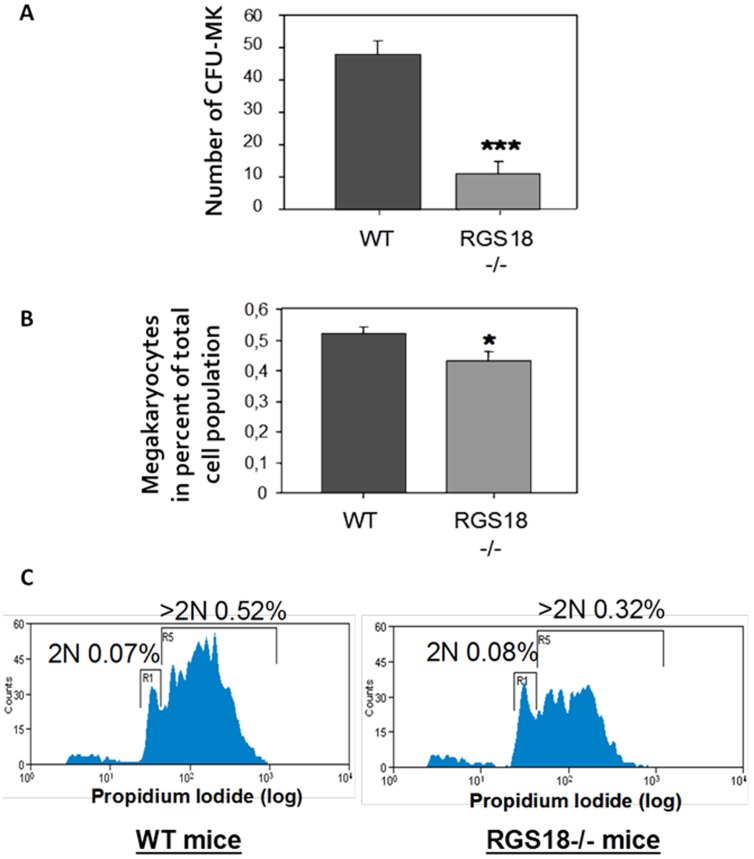
- Colonies containing >3 MKs were counted as CFU–MKs.
- Duplicate assays were performed for each mouse.
- At least two mice were analyzed for each sample group.