Human Interleukin-4 Recombinant (E.Coli)
Categories: HematopoietinsIL-2 familyRecombinant Human Cytokines$70.00 – $4,700.00
Description
Accession
P05112
Source
Optimized DNA sequence encoding Human Interleukin-4 mature chain was expressed in E.Coli
Molecular weight
Native human Interleukin-4, generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated molecular mass of approximately 15 kDa. Recombinant Interleukin-4 is a monomer protein consisting of 130 amino acid residue subunits, migrates as an approximately 15 kDa protein under non-reducingand reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation of human TF-1 cells is ≤.1 ng/ml, corresponding to a specific activity of >x units/mg.
Protein Sequence
MGLTSQLLPP LFFLLACAGN FVHGHKCDIT LQEIIKTLNS LTEQKTLCTE LTVTDIFAAS KNTTEKETFC RAATVLRQFY SHHEKDTRCL GATAQQFHRH KQLIRFLKRL DRNLWGLAGL NSCPVKEANQ STLENFLERL KTIMREKYSK CSS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Interleukin-4 was lyophilized from a 0.2 μm filtered solution in.5% glycine,.5% sucrose,.01% Tween80, mM Glutamic acid, pH.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Biological Process
Molecular function
Molecular function
Methods
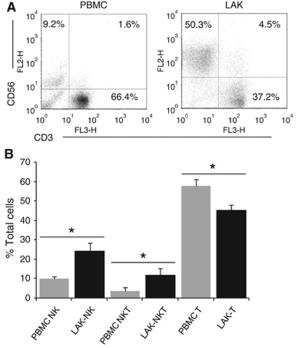
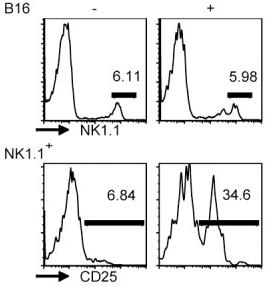
Phenotype of clinical grade LAKs and DCs.
- DCs were generated by culture of CD14+ PBMCs with GMCSF and IL-4 for 5 days.
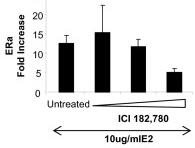
ICI 182,780 revert E2-inhibitory effect in the release of a-defensins 1-3 by DCs.
- CD14+ cells isolated from fresh blood were cultured for 5 days in the presence of IL-4 and GM-CSF to differentiate into DCs.
Isolation and culture of cells
- The adherence monocytes were cultured with RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, , ), 50 ng/ml human IL-4 , and 50 ng/ml human granulocyte-macrophage colony-stimulating factor (GM-CSF) at 37°C and 5% CO2.
- IL-4 and GM-CSF were added to the cultured cells every other day.
- After 5 days of culture, more than 95% of the cells had converted to immature HMDCs (iHMDCs), with a phenotype of CD11c+, CD54 low, CD83 low, CD40 low, CD58 low, CD86low, and CD80low, as determined by flow cytometry.
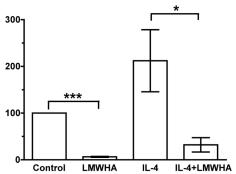
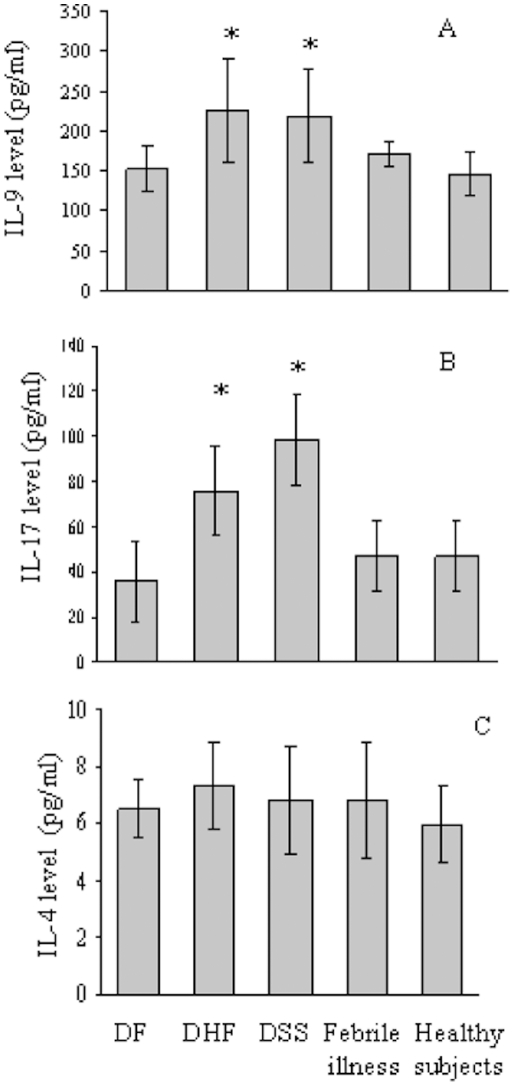
LMWHA inhibits IL-4 or IL-13-induced fibrocyte differentiation.
- Human PBMC were cultured in SFM, SFM with 1 ng/ml IL-4, SFM with 300 µg/ml LMWHA, or SFM with 1 ng/ml IL-4 and 300 µg/ml LMWHA, or SFM, SFM with 1 ng/ml IL-13, or SFM with 1 ng/ml IL-13 and 300 µg/ml LMWHA.
Generation of expanded DCs
- Conventional DCs were obtained from peripheral blood CD14+ cells as described previously, with minor modification.
- Briefly, CD14+ cells were cultured under GM/IL-4 (100 ng/ml GM-CSF and 50 ng/ml IL-4) in medium'>IMDM medium containing 10% FBS and 1% penicillin and streptomycin.
- CD14+ monocytes were obtained by negative selection.
- Cells were washed in PBS, and lineage antigen-positive (CD2, CD3, CD19, CD20, CD56, CD66b, CD123, Glycophorin A) cells were removed by using the EasySep human monocyte enrichment kit without CD16 depletion .
- These lineage-negative CD14+ cells were cultured under 100 ng/ml human GM-CSF and 50 ng/ml human IL-4 in IMDM medium.
- Each cytokine was dissolved in PBS containing 0.1% BSA in 100 times the density used.
- For expansion, CD34+ cells were cultured under GM/SCF (100 ng/ml GM-CSF and 50 ng/ml SCF) or GM/IL-4 (100 ng/ml GM-CSF and 50 ng/ml IL-4) in IMDM medium.
- The medium was…
Generation of expanded DCs
- Conventional DCs were obtained from peripheral blood CD14+ cells as described previously, with minor modification.
- Briefly, CD14+ cells were cultured under GM/IL-4 (100 ng/ml GM-CSF and 50 ng/ml IL-4) in medium'>IMDM medium containing 10% FBS and 1% penicillin and streptomycin.
- CD14+ monocytes were obtained by negative selection.
- Cells were washed in PBS, and lineage antigen-positive (CD2, CD3, CD19, CD20, CD56, CD66b, CD123, Glycophorin A) cells were removed by using the EasySep human monocyte enrichment kit without CD16 depletion .
- These lineage-negative CD14+ cells were cultured under 100 ng/ml human GM-CSF and 50 ng/ml human IL-4 in IMDM medium.
- Each cytokine was dissolved in PBS containing 0.1% BSA in 100 times the density used.
- For expansion, CD34+ cells were cultured under GM/SCF (100 ng/ml GM-CSF and 50 ng/ml SCF) or GM/IL-4 (100 ng/ml GM-CSF and 50 ng/ml IL-4) in IMDM medium.
- The medium was…
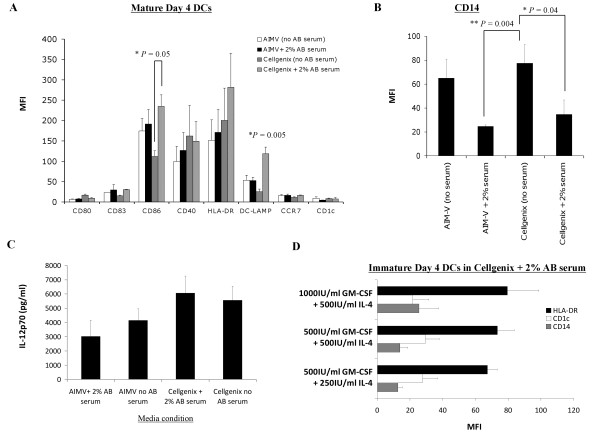
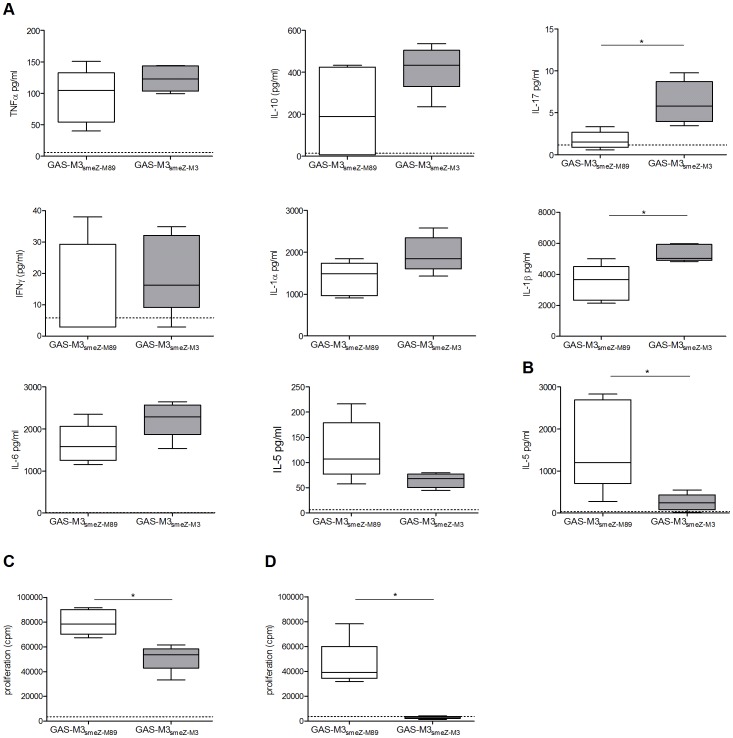
Comparison of the phenotype and IL-12p70 production of mature HOCl-oxidized lysate-loaded DCs generated in human AB serum-free or serum-containing AIM-V and CellGenix DC media, and supplementation with different concentrations of GM-CSF and IL-4.
- 500 IU/ml recombinant GM-CSF and 250 IU/ml recombinant IL-4 were sufficient concentrations for generating a favorable immature DC phenotype with low CD14, and intermediate HLA-DR and CD1c.
2.3. Differentiation of hESC
-
H1 hESCs were plated at 3 × 106 per well of 6-well ultralow attachment (ULA) plates in a total volume of 4 mL of medium'>X-VIVO-15 medium , supplemented with 1 mM sodium pyruvate, nonessential amino acids, 2 mM L-glutamine (all PAA Laboratories GmbH) and 5
μ M 2-mercaptoethanol . - The following growth factors were added: 50 ng/mL recombinant human bone morphogenetic protein-4 (BMP-4, & ), 50 ng/mL recombinant human vascular endothelial growth factor , 20 ng/mL recombinant human stem cell factor , and 50 ng/mL recombinant human granulocyte macrophage-colony stimulating factor (GM-CSF, & ).
- After 2-3 days, the medium was topped up with 2 mL of fresh supplemented X-VIVO-15 medium to produce a total volume of 6 mL.
- Subsequent feeding was performed every 2-3 days by replacing 2-3 mL of old medium with new supplemented X-VIVO-15 medium from which every 5 days a growth factor was removed starting with BMP-4 at day 5, followed by VEGF at…
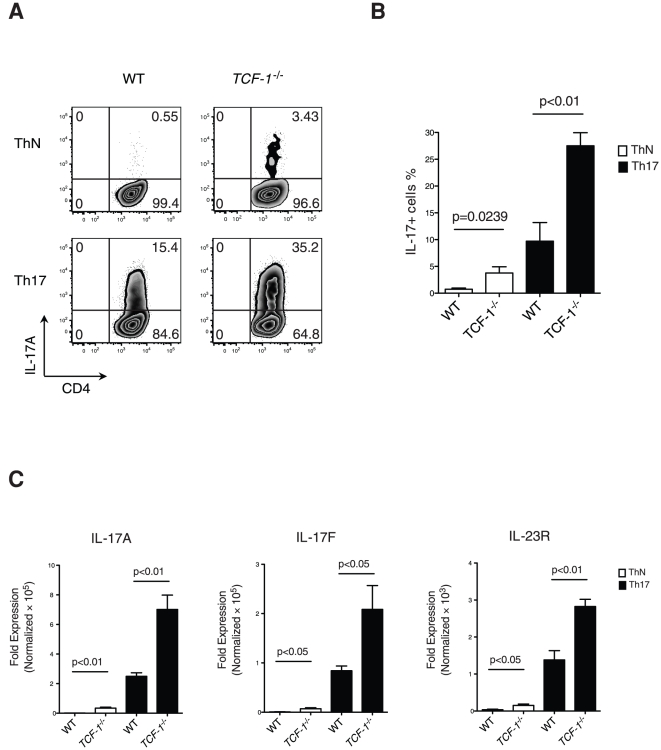
2.5. DC Maturation and Rapamycin Treatment
- Two days after monocytes were plated, monocyte-derived and hESC-derived immature DC were treated with 10 ng/mL and 5–7 ng/mL of rapamycin , respectively.
- On day 5, Cs were matured for 48 hr using a maturation cocktail consisting of 50 ng/mL of GM-CSF , 100 ng/mL IL-4 , 20 ng/mL IFN
γ , 50 ng/mL TNFα , 10 ng/mL of IL-1β , and 1μ g/mL PGE2 . - On day 6-7, DCs were harvested by gentle pipetting, passed through a 70
μ m cell strainer, centrifuged, and resuspended prior to their use in experiments.
Generation of imDC In Vitro
- Monocytes and CD3+ T cells were isolated from PBMCs by using anti-CD14 and anti-CD3 microbeads respectively [+ cells and CD3+ T cells was consistently ranging from 93% to 97%, as determined by flow cytometry.
- Monocytes were cultured in RPMI supplemented with 10% human AB serum, 10 ng/ml IL-4, and 50 ng/ml GM-CSF for 7 days to generate imDC as we described before [