Human Interleukin-21 Recombinant
Categories: HematopoietinsIL-2 familyRecombinant Human Cytokines$70.00 – $4,700.00
Description
Accession
Q9HBE4
Source
Optimized DNA sequence encoding Human Interleukin-21 mature chain was expressed in Escherichia Coli.
Molecular weight
Human Interleukin-21 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 15kDa. Recombinant IL-21 is a monomer protein consisting of 132 amino acid residue subunits, and migrates as an approximately 15kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent proliferation of activated B Cells.
Protein Sequence
MERIVICLMV IFLGTLVHKS SSQGQDRHMI RMRQLIDIVD QLKNYVNDLV PEFLPAPEDV ETNCEWSAFS CFQKAQLKSA NTGNNERIIN VSIKKLKRKP PSTNAGRRQK HRLTCPSCDS YEKKPPKEFL ERFKSLLQKM IHQHLSSRTH GSEDS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Interleukin-21 was lyophilized from a 0.2 μm filtered PBS solution pH7.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Molecular function
Methods
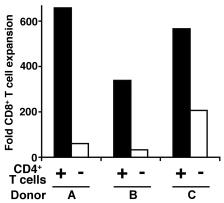
Autologous CD4+ T cell secretion of IL-2/IL-21 is necessary but not sufficient to help CD8+ T cells proliferate.
- IL-2, IL-21, or both were added in each condition.
Mixed lymphocytes tumor cell culture (MLTC)
- PBMCs isolated from melanoma patients were thawed and incubated overnight at 37°C.
- PBMCs (106 cells/well) were cultured in the presence of autologous irradiated (150 Gy) melanoma cells at a lymphocyte to tumor ratio of 5:1 in 24-well plates with X-VIVO15 and 5% HS.
- Different cytokine combinations were added to these MLTCs in order to select the most suitable in vitro culture conditions in terms of T lymphocyte expansion and efficiency in tumor cell recognition: (1) 120 IU/ml rhIL-2 ; (2) 120 IU/ml rhIL-2 and 10 ng/ml rhIL-15 ; (3) 10 ng/ml rhIL-15; (4) 5 ng/ml rh-IL-7 ; (5) 120 IU/ml rhIL-2 and 5 ng/ml rhIL-7; (6) 120 IU/ml rhIL-2 and 10 ng/ml rhIL-21 ; (7) 10 ng/ml rhIL-21 alone.
- Fresh medium containing the above indicated cytokines was replaced every 3 days.
- These MLTCs were stimulated weekly with irradiated autologous melanoma cells, and their reactivity was tested starting from the 3rd week of culture.
DC Co-Cultures
- Sorted naïve (live, singlet, CD3+CD28+CD95-CCR7+CD27+) CD4+ T cells were co-cultured with sorted CD103+ DCs (live, lineage-CD14-HLA-DR+CD11c+) (100:1), sorted CD103− DCs (lineage-CD14-HLA-DR+CD11c+CD103-) (100:1), or anti-CD3/anti-CD28 beads (control, 4:1) in X-Vivo15 media under Th17 conditions (10 U/mL recombinant human IL-2, 12.5 ng/mL rhIL-1β, 25 ng/mL rhIL-21, 25 ng/ml rhIL-23, 10 μg/mL anti-IFNγ, 10 μg/mL anti-IL-12 and 2 ng/mL TGF-β, with or without 25 ng/mL rhIL-6).
- All cytokines were from , except IL-2, IL-6 and IL-21 .
- DC co-cultures were stimulated with SEB (1 μg/mL ).
- All cultures were fed on day 3 with rhIL-2 (2 U/mL), anti-IFNγ (10 μg/mL) and anti-IL-12 (10 μg/mL).
- After 7 days of co-culture, qRT-PCR was performed for
IL17A andRORc gene expression . - Gene expression was quantified using ΔΔCT analysis in excel (version 12.3.0).
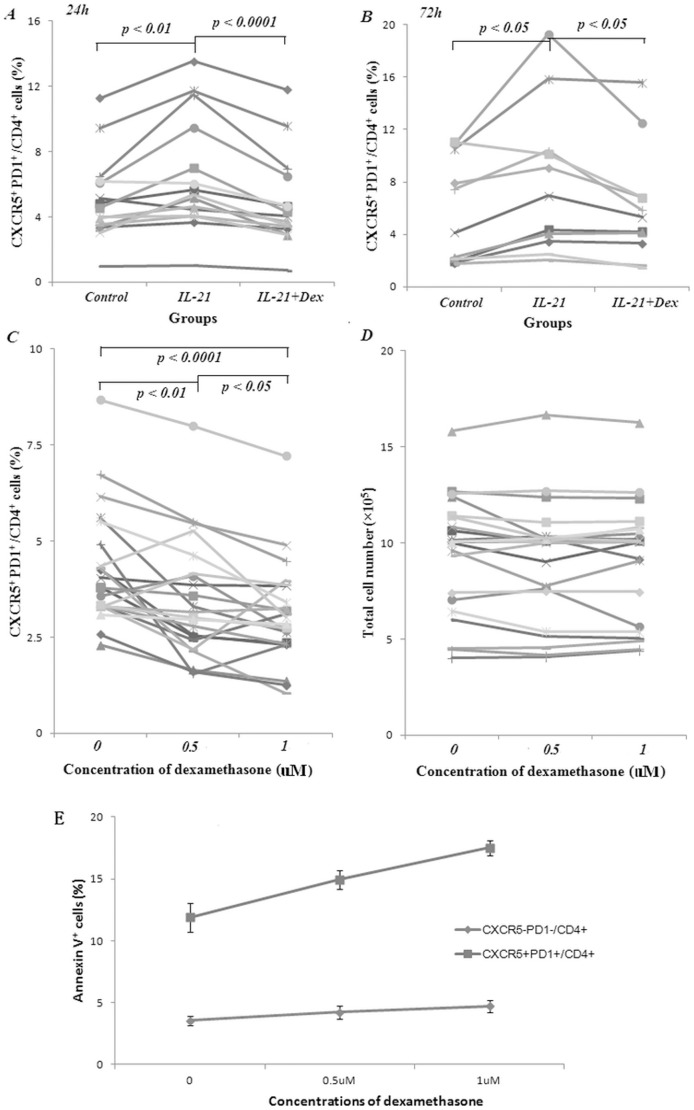
Decreased CXCR5+ PD1+/CD4+ cell production by dexamethasone.
- A. Dexamethasone (Dex) reduced the proportion of IL-21 stimulated CXCR5+ PD1+/CD4+ cell.
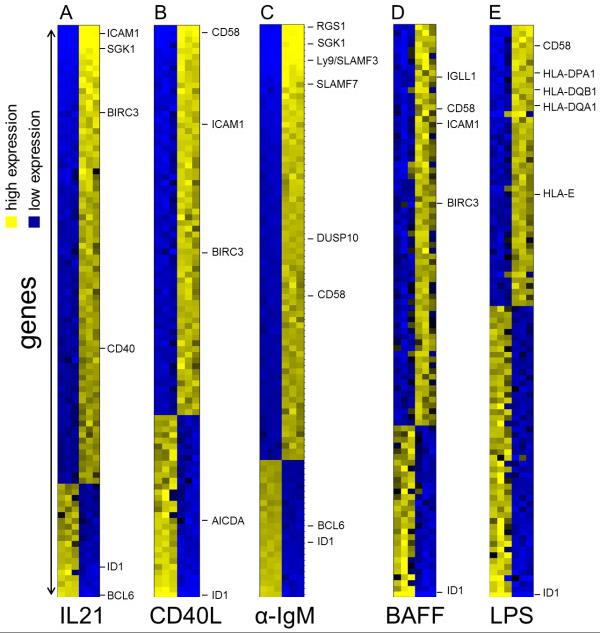
Identification of IL21, CD40L, αIgM, BAFF and LPS regulated genes in transformed human germinal centre B cells using microarrays.
- BL2 cell were stimulated with αIgM F(ab)2 fragments (3 hrs) , IL21 (2 hrs) , CD40L (6 hrs) , LPS (6 hrs) and BAFF (9 hrs) .
In vitro Sensitization (IVS) of CD8+ T Cells
- CTLs were induced as previously described + donors were pulsed with tumor cell lysates for the last 24 h of maturation.
- Based on the number of tumor cells before lysis, tumor cells were added to DC at a 3∶1 ratio.
- TA-pulsed mDC were then irradiated (3000 rad) and washed with PBS.
- Autologous CD8+ T cells were isolated from cryopreserved PBMC by negative selection using magnetic bead separations and added to the mDC at the 10∶1 ratio.
- Cells were cultured in an atmosphere of 5% CO2 in air at 37°C for 7 days in AIM-V media containing 10 ng/ml IL-7 and 10 ng/ml IL-21 and 5% (v/v) FBS.
- On day 7, fresh, tumor cell lysate-pulsed and irradiated mDC were added and T cells were cultured for additional 7 days in AIM V containing 20 IU/ml IL-2 , 5 ng/ml IL-7, 10 ng/ml IL-21 and 5% (v/v) FBS.
- On day 14, cells were…
- B cells were obtained from peripheral blood of healthy volunteers by CD22 MACS microbead magnetic column selection .
- Purity was > 97% and was determined by flow cytometry by staining for CD19 and CD20.
- We co-cultured B cells (2 - 2.5 × 105 cells/ml) on irradiated (50 Gray) CD40L-expressing L cell fibroblasts (5 × 104 cells/ml, > 96% surface CD154+) in 1 ml Iscove's Modified Eagle's Medium containing 8% fetal bovine serum and penicilin/streptomycin and with or without rhIL-4 (25 ng/ml) or rhIL-21 (10 ng/ml) in 24-well plates.
- After 6 days, we harvested supernatants and the levels of IgG4 in the supernatant were determined as described below.