Human Interleukin-2 Recombinant
Categories: HematopoietinsIL-2 familyRecombinant Human Cytokines$70.00 – $720.00
Description
Accession
P60568
Source
Optimized DNA sequence encoding Human Interleukin-2 mature chain was expressed in Escherichia Coli.
Molecular weight
Native Human Interleukin-2 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 16kDa. Recombinant Interleukin-2 is a monomer protein consisting of 134 amino acid residue subunits, and migrates as an approximately 16kDa protein under non-reducing conditions and reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation of monkeyMBr-5 cells was found to be in the range of.0-40.0 ng/ml.
Protein Sequence
MYRMQLLSCI ALSLALVTNS APTSSSTKKT QLQLEHLLLD LQMILNGINN YKNPKLTRML TFKFYMPKKA TELKHLQCLE EELKPLEEVL NLAQSKNFHL RPRDLISNIN VIVLELKGSE TTFMCEYADE TATIVEFLNR WITFCQSIIS TLT
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Human Interleukin-2 was lyophilized from a 0.2 μm filtered PBS solution.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Biological Process
Molecular function
Molecular function
Methods
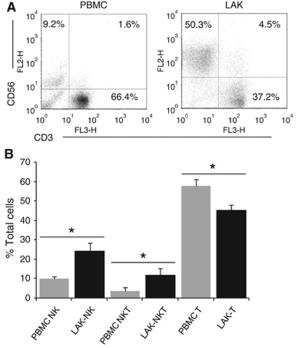
Phenotype of clinical grade LAKs and DCs.
- Phenotype analysis was performed on CD14− PBMCs before and after culture for 5 days in IL-2 to generate LAKs.
Derivation of γδ T Cells
- Human peripheral blood was collected (30 ml) from adult healthy donors after obtaining the IRB approval from the Ohio State University Medical Center and obtaining written consents from donors.
- The ethic committee has also approved the procedure and records are saved in the laboratory logbook.
- Freshly collected blood was processed to isolate peripheral blood mononuclear cells (PBMC) following the similar protocol published earlier
2.5. Transduction of PBMCs and T Cells
- Peripheral blood monocytes (PBMCs) were from an HLA-A2, healthy human.
- T cells were obtained from anti-CD3 conjugated magnetic beads .
- The PBMCs and T cells were cultured in AIM-V and interleukin-2 (IL-2; PeproTech, , , ) at 300 IU/mL.
- For medium'>OKT3 stimulation, the cells were placed initially in either a medium with anti-CD3 antibody, medium'>OKT3 at 50 ng/mL or in an medium'>OKT3 medium after transduction at the initial changing of the culture medium in the presence of IL-2.
- For transduction of the PBMCs or T cells, 1 × 106 cells were adjusted to a final volume of 1 mL in a 24-well, tissue culture-treated plate with the viral supernatant and(8 mg/mL, , , ).
- The cells were transduced by centrifugation of the plates at 1000 g for 1.5 hours at 32°C.
- The plates were placed in a 37°C,…
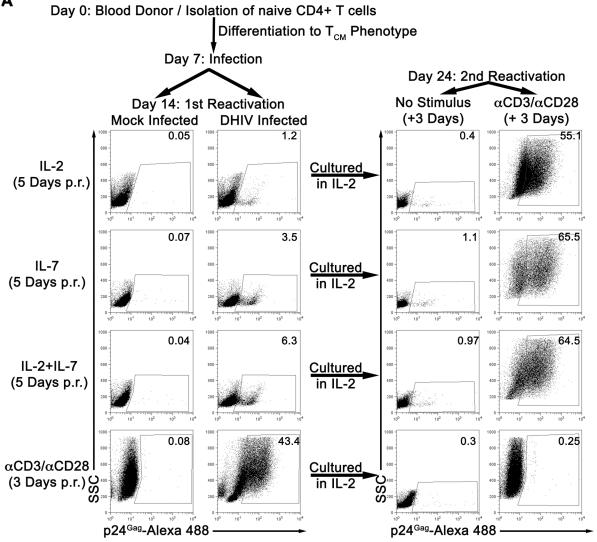
IL-7 induces partial reactivation of latent HIV-1 in cultured TCM.
- Mock or DHIV latently infected, cultured TCM were incubated in the presence of IL-2, IL-7 or a combination of IL-2 and IL-7 (IL-2+IL-7) or costimulated with antibodies to CD3 and CD28 (αCD3/αCD28) and assessed for intracellular p24Gag by flow cytometry (1st Reactivation).
Cell isolation and culture
- Peripheral blood mononuclear cells (PBMC) from CLL patients or healthy donors were separated from heparinized venous blood by Ficoll density gradient centrifugation.
- The PBMC used in this study were generally greater than 90% CLL B cells as determined by CD19/CD5 positivity.
- Leukemic cells were cultured in serum-free adoptive immunotherapy media-V medium'>(AIM-V) medium .
- For CLL B cell activation experiments, cells were cultured for 5 days in AIM-V media or AIM-V media supplemented with 2.5 µg/ml CpG ODN 2006 (provided by core Mayo Clinic facility) with or without 94 IU/ml human IL-2 , 10 ng/ml human IL-15 , and 13 µM beta-mercaptoethanol.
- Further CLL B cell activation experiments utilized 12-O-tetradecanoylphorbol-13-acetate (TPA) at 10 ng/ml or interferon-α 2b at 1000 IU/ml.
- Human C4+T cells were isolated from healthy donor PBMC by immunomagnetic selection using T cell enrichment kits and incubated with CLL B cells…
2.4. Coculture of CHO/IDO Cells and CD3+ T Cells
- The CD3+ T cells in peripheral blood mononuclear cells (PBMCs) of breast cancer patients were purified using Human Pan T-cell Isolation Kit II according to the manufacturer's instructions.
- 1 × 105 CHO/IDO cells and CHO/EGFP cells were seeded in a 24-well plate and cocultured with 2 × 106 purified T cells in complete RPMI 1640 medium supplemented with 10% FBS and 50 U/mL rhIL-2 at 37°C in a 5% CO2 incubator.
- Unstimulated T cells cocultured in complete RPMI 1640 medium supplemented with 10% FBS and 50 U/mL rhIL-2 were used as control.
- The nonadherent T cells under different treatments were harvested 7 days later for flow cytometry analysis, quantitativee real time RT-PCR, and Western Blot analysis.
Stimulation of antiviral T cells with HSP70, CMVpp65495-503 peptide, and HSP70/CMV-PC
- T-cell stimulation was performed using samples from 16 A2/CMV-pentamer-positive donors.
- Peripheral blood mononuclear cells (PBMCs) were isolated by discontinuous gradient centrifugation, washed twice in sterile PBS, resuspended at a concentration of 1 × 107 cells/ml in medium'>RPMI1640 culture medium , and then supplemented with 10% heat-inactivated human AB serum (C.C.pro, Neustadt, ) and 100 U/ml IL-2 .
- Cells were stimulated in a 24-well plate with either 10 μg/ml HSP70, 10 μg/ml CMVpp65495-503 peptide, or 10 μg/ml HSP70/CMV-PC.
- HSP70-peptide-binding buffer was used as negative control.
Retroviral delivery of MART-1 specific TcRs.
- IL-2 release was monitored by ELISA assay, and plotted as pg of IL-2 per mL of medium.
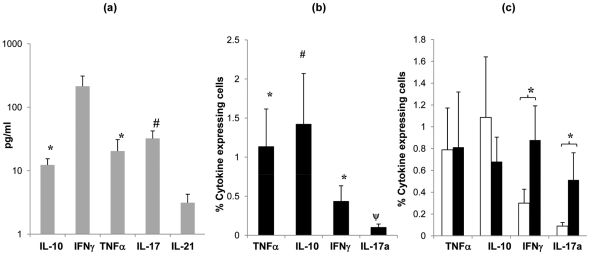
Cytokine profile of tonsil MNCs following S. pneumoniae antigen stimulation.
- Cytokine (IL-10, IFNγ, TNFα, IL-2 and IL-17) production by tonsil MNCs following stimulation with Ply (grey bars, SEM are shown by error bars)) was assessed by quantifying cytokine levels in cell culture supernatants (n = 8) at 7 days post cell stimulation by Luminex assay.
Ex vivo culture of human peripheral blood T cells
- For regulatory T cell culture, human enriched T cells were co-cultured with MSCs or SB623 cells in the presence of human interleukin-2 (IL-2) at a 10:1 T cells to MSC or SB623 cell ratio for 7 days followed by cell surface staining for CD4, a helper T cell marker and CD25, the IL-2 receptor alpha chain.
- For FoxP3 intracellular staining, cells were fixed and permeabilized with CytoFix/Perm (eBioscience).
- PE-conjugated antibody against FoxP3 (clone PCH101, eBioscience) was used at 1:50 dilution and flow cytometry analysis was done gating on lymphocytes.
- For assessment of constitutive IL-10 production, intracellular staining with fluorochrome conjugated antibody against IL-10 was performed without PMA/Io stimulation on7.