Human FLT3 Ligand Recombinant
Category: Recombinant Human Cytokines$70.00 – $3,500.00
Description
Accession
P49771
Source
Optimized DNA sequence encoding Human FLT-3 Ligand mature chain was expressed in Escherichia Coli.
Molecular weight
Native human FLT3 Ligand is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 17 kDa. Recombinant FLT-3 Ligand is a monomeric protein consisting of 156 amino acid residue subunits, and migrates as an approximately 17 kDa protein under non-reducing conditions and reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation of Human AML5 cells was found to be in the range of 0.2 ng/ml.
Protein Sequence
MTVLAPAWSP TTYLLLLLLL SSGLSGTQDC SFQHSPISSD FAVKIRELSD YLLQDYPVTV ASNLQDEELC GGLWRLVLAQ RWMERLKTVA GSKMQGLLER VNTEIHFVTK CAFQPPPSCL RFVQTNISRL LQETSEQLVA LKPWITRQNF SRCLELQCQP DSSTLPPPWS PRPLEATAPT A PQPPLLLLL LLPVGLLLLA AAWCLHWQRT RRRTPRPGEQ VPPVPSPQDL LLVEH
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant FLT-3 Ligand was lyophilized from a 0.2 μm filtered PBS solution pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Molecular function
Methods
Processing whole blood samples
- Peripheral (PB.1 and PB.2) and cord (CB.1 and CB.2) blood-derived CD34+ cells were obtained from AllCells .
- Blood collections were performed at AllCells and using standard, 8 ml Vacutainer Cell Processing Tubes (both sodium citrate and sodium heparin-based tubes are acceptable ; , ).
- Appropriate documentation for informed consent was completed prior to blood collection .
- Vacutainers were processed within 24 hours of collection.
- Briefly, the PBMC-containing upper phase was collected and washed with ice-cold PBS .
- Cells were either frozen down or used directly for purification with the CD34 MicroBead Kit and used according to the manufacturer's protocol.
- Some samples were treated with Histopaque ( ; St. Louis, ) to minimize the number of red blood cells and centrifuged at 2000 rpm for 20 minutes without braking.
- The interface containing the PBMCs was removed if samples were treated with histopaque, cells washed again with chilled…
Cell cultures
- Fresh UCB was obtained from , and the use of the UCB samples was reviewed and approved by the institutional review boards (IRB) of each cord blood collection hospital as well as those of the and National University of (NUS).
- The UCB-MNC were isolated using Ficoll Histopaque-1077 density-gradient centrifugation and counted before cyropreservation in 90% v/v donor autoplasma with 10% v/v dimethyl sulfoxide (DMSO ) for subsequent use.
- CD34+ selected cells were obtained using Magnetic Activated Cell Sorting (MACS) cell-separation columns (Milte-nyi Biotec GmbH, Bergisch Gladbach, Germany).
- The cryopreserved UCB-MNC were thawed using a thawing solution containing human albumin (HAS; 20% w/v , ) and Onkovertin 40 (10% w/v; B. Braun, ).
- The cells were centrifuged at 400
g for 15 min at 10 ° C. The cells were then washed with Dulbecco's phosphate-buffered saline , followed by centrifugation at 300g for another…
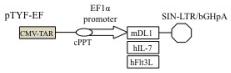
Enhanced proliferation of T cell precursors on LmDL1-FL7., Lentivector constructs expressing mouse DL1, human IL-7 and human Flt3L.
- , qRT-PCR analysis for DL-1, Flt3L, and IL-7.
Cell lines and cell culture
- 293T ( .
- number CRL-11268) and NIH3T3 ( .
- number CRL-1658) cells were both cultured in medium'>Dulbecco's medium'>modified medium'>Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1% Penicillin-Streptomycin with 5% CO2 at 37°C.
- Bone marrow cells (BM) isolated from wild type (wt) C57BL/6 mice were cultured in StemSpan Serum-free expansion medium supplemented with 1% glutamine, 1% penicillin-streptomycin, 100 ng/mL each murine stem cell factor , human Flt3-ligand and human interleukin 11 , and 10 ng/mL murine interleukin 3 .
- The KSHV-positive PEL cell line BCBL-1
Generation of human UCB/CD34+ cell-derived NK cells
- Human UCB/CD34+ cell-derived NK cells were prepared according to a modified protocol originally described previously+ cells (1 × 105 cells per well) were cultured with GMP Serum-free Stem Cell Growth Medium (CellGro/CellGenix) supplemented with 30 ng ml−1 SCF and 50 ng ml−1 Flt3-L .
- IL-15 (50 ng ml−1) and/or IGF-1 (100 ng ml−1) were added to SCF/Flt3-L-containing cultures as specified in the figure legends.
- The plates were incubated for 4 weeks at 37 °C in a humidified atmosphere with 5% CO2.
- Half of the medium volume was replaced with fresh medium and cytokines twice every week.
Preparation of dendritic cells
- BMDCs were generated from 6- to 8-week-old female C57BL/6J, DBA/2J and BXD mice .
- All animal protocols were reviewed and approved by the MIT / Whitehead Institute / Broad Institute Committee on Animal Care (CAC protocol 0609-058-12).
- Bone marrow cells were collected from femora and tibiae and plated at 106 cells/ml on non-tissue culture treated Petri dishes in medium'>RPMI-1640 medium , supplemented with 10% FBS, L-glutamine, penicillin/streptomycin, MEM nonessential amino acids, sodium pyruvate, β-mercaptoethanol, and murine GM-CSF (15 ng/ml) or human Flt3L (100 ng/ml).
- For microarray experiments, floating cells from GM-CSF cultures were sorted at day 5 by MACS using the CD11c (N418) MicroBeads kit .
- Sorted CD11c+ cells were used as GM-CSF-derived BMDCs, and plated at 106 cells/ml and stimulated at 16 hr post sorting.
- For all other experiments, GM-CSF-derived BMDCs were used directly and composed of ~90% CD11c+ cells,…
Cell Culture
- CD34+ bone marrow cells were received fresh and were plated and expanded immediately upon receipt.
- Cells were expanded for 3–5 days in StemSpan SFEM before being passaged for media formulation experiments.
- StemSpan SFEM media was supplemented with 100 ng/mL SCF, 100 ng/mL FLT3, 25 ng/mL IL6, and 25 ng/mL IL3 during expansion.
- Human embryonic stem cell line SA181 (Cellartis AB, Goteborg, Sweden) was cultured on matrigel coated flasks in medium'>TesR2 medium during expansion.
- ESCs were split for reprogramming experiments using TrypLE .
- Human adult female cryopreserved hepatocytes ( .
- F00995, Celsis/In Vitro Technologies, Chicago, IL) were thawed in medium'>CHRM medium and plated in Williams medium E supplemented with Primary Hepatocyte Thawing and Plating Supplement Pack .
- BJ fibroblasts and MRC-5 fibroblasts were thawed and plated directly into DMEM/F12+Glutamax with…
Mice, Cell culture and primary cell samples
- The knockout mouse models of Jmjd3 and Utx as well as the corresponding genotyping strategy have been described in published studies.
- All animals used in this study were treated according to IACUC protocols for, and .
- The human T-ALL CUTLL11, P12-Ichikawa, Loucy, DND41, CEM, Jurkat and myeloid leukemia (THP1, HL-60) lines as well as the mouse T-ALL line (“720”) were all cultured in RPMI 1640 medium supplemented with 20% FBS and penicillin, streptomycin.
- All cell lines were being tested for the presence of mycoplasma and only mycoplasma-free lines were used for these studies.
- Primary human samples were collected by collaborating institutions with inform consent and analyzed under the supervision of the Columbia University Medical Center and St Jude Children’s Hospital Institutional Review Board.
- The primary cells treated with GSKJ4 inhibitor (for more information on these cells please check
King et al .) were cultured in MEMα medium plus 10% fetal bovine…
-
CD34 Cord blood (CB) samples were obtained from the Hannover Medical School following written consent of the donors as approved by the Hannover Medical School local ethics committee. - Total nucleated cells were isolated by a Ficoll gradient followed by enrichment for CD34+ cells employing MACS purification .
- Isolated cells were frozen until further usage.
- CB-CD34+ cells were cultured in medium'>StemSpan medium supplemented with 1% penicillin/streptomycin and one of four cytokine combinations using hSCF, hTHPO, hFLT3-L, hGCSF, hIGFBP2, hAngptl5, hFGF-1, and hIL6 .
- The StemRegenin compound was used at a concentration of 1 µmol/l as described.
Cell Preparation and Cell Culture
- Human UCB was obtained from donors after informed consent according to the Declaration of Helsinki.
- The experimental usage of UCB samples was approved by the local ethics commission.
- Mononuclear cells (MNCs) were isolated from individual sources by Ficoll density gradient centrifugation and highly enriched by magnetic cell separation as described previously (2 at densities of 0.5–1 × 105 cells/ml in’s modified medium'>Dulbecco’s medium supplemented with 20% fetal bovine serum , 100 U/ml penicillin, and 100 U/ml streptomycin and with early-acting cytokines (FLT3L, stem cell factor, and thrombopoietin, each at 10 ng/ml final concentration [all]).