Human Fibroblast Growth Factor 10 Recombinant
Categories: FGF familyFGF family$70.00 – $2,700.00
Description
Accession
O15520
Source
Optimized DNA sequence encoding Human Fibroblast Growth Factor-10 mature chain was expressed in Escherichia Coli.
Molecular weight
Native human FGF10 is generated by the proteolytic removal of the signal peptide and propeptide, this molecule has a calculated molecular mass of approximately 19 kDa. Recombinant Fibroblast Growth Factor is a monomer protein consisting of 170 amino acid residue subunits,and migrates as an approximately 19 kDa protein reducing conditions in SDS-PAGE.
Purity
>96%, as determined by SDS-PAGE and HPLC
Biological Activity
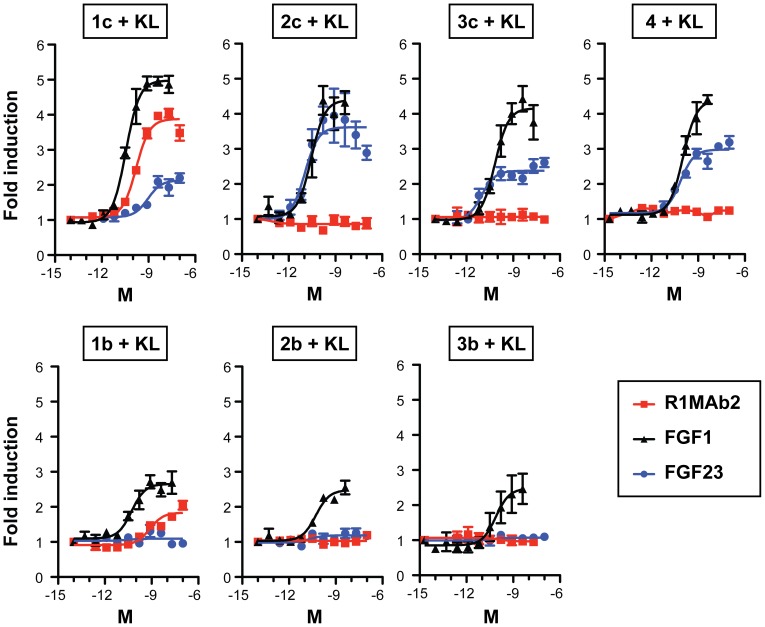
The ED(50) was determined by the dose-dependent proliferation of BaF3 cells expressing FGF receptors, and was found to be <0.5ng/ml., corresponding to a specific activity of.0 x Units/mg.
Protein Sequence
QAL GQDMVSPEAT NSSSSSFSSP SSAGRHVRSY NHLQGDVRWR KLFSFTKYFL KIEKNGKVSG TKKENCPYSI LEITSVEIGV VAVKAINSNY YLAMNKKGKL YGSKEFNNDC KLKERIEENG YNTYASFNWQ HNGRQMYVAL NGKGAPRRGQ KTRRKNTSAH FLPMVVHS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
RecombinantFGF10 was lyophilized from.2 μm filtered PBS solution, pH7.0 .
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Molecular function
Methods
Endothelial Sprouting Assay
- Endothelial sprouting assay was performed as described 4 ESC were seeded in 35 mm dishes in 1.2 mg/ml collagen, type I neutralized with 0.1 N NaOH.
- Collagen media contained 15% FBS, 450 µm MTG, 10 µg/ml insulin , 50 ng/ml human vascular endothelial growth factor (VEGF, , ), 100 ng/ml human basic fibroblast growth factor (FGF-2, , ) in IMDM.
- Cells were incubated at 37°C, 5% CO2.
- At day 6, 200 µl media without collagen was added and EB were analyzed on day 11.
- Image analysis was performed using AxioVision LE Software .
Materials
- Fetal bovine serum (FBS), Dulbecco's Modified Eagle Medium (DMEM), Penicillin–Streptomycin (Pen–Strep), HEPES and trypsin/EDTA were obtained from .
- Ascorbic acid-2-phosphate, dexamethasone, sodium-β-glycerophosphate, Triton X-100, fibrinogen and thrombin were obtained from (St. Louis, ).
- Basic fibroblast growth factor (bFGF) and bone morphogenetic protein 2 (
BMP2 ) were obtained from . - Endothelial Growth Medium-2 (
EGM ) was obtained from . - Phosphate buffered saline (PBS) and proteinase K were obtained from Fisher Scientific .
- All other substances were of analytical or pharmaceutical grade and obtained from Sigma-Aldrich.
Cell culture
- Human CAPs were cultured at a density of 6000 cells/cm2 in medium consisting of equal amounts of IMDM (PAA)/DMEM/Ham's F12 medium containing 5% human serum, 1% penicillin/streptomycin, 20 ng/ml basic fibroblast growth factor and 10 ng/ml epithelial growth factor .
- Human cardiac fibroblasts were cultured in Lung/Cardiac Fibroblasts Basal Medium (Cell Applications, Inc. San Diego, USA) plus supplements (CELL Applications).
- CAR and CD55 expression flow cytometry analysis was performed on CAPs and cardiac fibroblasts of the same passage number.
- Murine HL-1 cells were cultured in Claycomb medium (SAFC Biosciences, Kansas, USA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 100 µM norepinephrine and 2 mM glutamine.
- Chinese hamster ovary (CHO) cells and CHO cells expressing human CAR (a kind gift of J.M.
- Bergelson, Children's Hospital of Philadelphia, Philadelphia) were cultured in Hams F12
Human mesenchymal stem cells (MSCs) isolation
- Human bone marrow (BM) was aspirated from the iliac crest of healthy donors.
- Fresh BM was cultured in flasks seeding 10 µl BM cells/cm2 with medium'>alpha-minimum medium'>essential medium (α-MEM) supplemented with 2 mM L-glutamine, 15% fetal bovine serum (FBS) , 100 U/ml Penicillin, 0.1 mg/ml Streptomycin and 1 ng/ml of fibroblast growth factor-basic (FGF-b , , ) 3 cells/cm2 and cultured under the same conditions.
Cell culture and sphere-forming assay
-
The sphere forming assay was performed as described previously by
. - with minor modifications [
HaCaT Cells
- HaCaT cells, an immortalized human keratinocyte line (kind gift of Dr P. Boukamp; 2.
- Cells were passaged weekly in T75 flasks and all the experiments were performed with sub-confluent cells.
- HaCaT cells at 70% confluence were stimulated with hFGF7, hFGF10 or hFGF22 at a concentration of 100 ng/ml, co-treated with(300 ng/ml).
- FGF dependent stimulation was blocked by 30 minute pre-treatment with the FGFR specific inhibitor, PD173074 (1.7 µM).
- MSCs were placed into step one medium, which consists of DMEM supplemented with SPN, 2mM L-glutamine, 20ng/ml human epidermal growth factor (hEGF, R&D Systems, Minneapolis, MN, ), 20ng/ml human basic fibroblast growth factor (hbFGF, R&D Systems) and 10μL/ml N2 supplement (insulin 5μg/ml, progesterone 20nM, putrescin 100μM, selenium 30nM, transferrin 100μg/ml, , , ).
- 72 hours later, the MSCs were placed into step two medium consisting of DMEM supplemented with SPN, 2mM L-glutamine, 1mM dibutyryl cyclic AMP (dbcAMP), 0.5mM isobutylmethylxanthine (, from), 20 ng/ml hbFGF, 50ng/ml human neuregulin1-β1 and 5 ng/ml Platelet-Derived Growth Factor-AA (PDGF).
- MSCs grown in medium'>serum-free medium containing DMEM, glutamine and SPN served as untreated controls.
- Due to species-specific toxicity, when inducing mouse MSCs the concentrations of IBMX and cAMP were halved in the step two medium.
Cell culture, staining, and imaging
-
Satellite cells were identified by FACS and single cells were deposited into 96-well plates in myogenic medium: DMEM/F12 medium without
l -glutamine (Cell Gro, Manassas, VA; 15-090-CV) containing 20% FBS , 10% horse serum (26050-088), 50 ng/μL human basic fibroblast growth factor (, , 100-18), 1% penicillin/streptomycin (15140-122), 1% Glutamax , and 0.5% chick embryonic extract (US , , ; ).
Cell culture
- The human retinoblastoma cell line, WERI-Rb1, obtained from the American Type Culture Collection , was maintained in RPMI-1640 Medium with 10% fetal bovine serum (FBS, , ) [2 and observed under inverted microscopy every other day.
Generation and administration of UC-MSCs
- UCs (n = 10, clinically normal pregnancies, approved by the Qilu hospital’s human research ethics committee) were excised and washed in a 0.1 mol/l phosphate buffer (pH 7.4) to remove excess blood.
- The cords were dissected and the blood vessels were removed.
- The remaining tissues were cut into small pieces (1–2 mm3) and placed in plates with low-glucose medium'>ulbecco-modified medium'>Eagle medium (L-MEM) , supplemented with 10% fetal bovine serum (FBS), 2 ng/mL vascular endothelial growth factor (VEGF& , , ), 2 ng/mL epidermal growth factor (EGF& ), 2 ng/mL fibroblast growth factor (FGF& ), 100 U/ml penicillin, and 100 μg/ml streptomycin .
- Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2.
- The media were changed every 3–4 d. Adherent cells proliferated from individual explanted tissues 7–12 d after initiating incubation.
- At this time, the small tissue pieces were removed from the culture and the adherent fibroblast-like…