Human Platelet Derived Growth Factor-BB Recombinant
Categories: PDGF familyPDGF familyRecombinant Human Cytokines$70.00 – $3,500.00
Description
Accession
P01127
Source
Optimized DNA sequence encoding Human PDGF-BB mature chain was expressed in Saccharomyces cerevisiae.
Molecular weight
Human PDGF-BB, generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately kDa . Recombinant PDGF-BBis a disulfide-linked homodimeric protein consisting of two 109 amino acid residue subunits. Recombinant PDGF-BB migrates due to glycosylation as an approximately 32 kDa protein under non-reducing conditions and as 15kDa under reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC.
Biological Activity
The ED(50) was determined by the dose-dependent proliferation of Balb/cT3 cells.The expected ED50 for this effect is 1 ng/ml, corresponding to 1x10^6 IU/mg.
Protein Sequence
MNRCWALFLS LCCYLRLVSA EGDPIPEELY EMLSDHSIRS FDDLQRLLHG DPGEEDGAEL DLNMTRSHSG GELESLARGR RSLGSLTIAE PAMIAECKTR TEVFEISRRL IDRTNANFLV WPPCVEVQRC SGCCNNRNVQ CRPTQVQLRP VQVRKIEIVR KKPIFKKATV TLEDHLACKC ETVAAARPVT RSPGGSQEQR AKTPQTRVTI RTVRVRRPPK GKHRKFKHTH DKTALKETLG A
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant PlateletDreived GrowthFactor-BB was lyophilized from a 0.2 μm filtered.02M acetic acid solution pH.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Molecular function
Molecular function
Molecular function
Methods
- MSCs were placed into step one medium, which consists of DMEM supplemented with SPN, 2mM L-glutamine, 20ng/ml human epidermal growth factor (hEGF, R&D Systems, Minneapolis, MN, ), 20ng/ml human basic fibroblast growth factor (hbFGF, R&D Systems) and 10μL/ml N2 supplement (insulin 5μg/ml, progesterone 20nM, putrescin 100μM, selenium 30nM, transferrin 100μg/ml, , , ).
- 72 hours later, the MSCs were placed into step two medium consisting of DMEM supplemented with SPN, 2mM L-glutamine, 1mM dibutyryl cyclic AMP (dbcAMP), 0.5mM isobutylmethylxanthine (, from), 20 ng/ml hbFGF, 50ng/ml human neuregulin1-β1 and 5 ng/ml Platelet-Derived Growth Factor-AA (PDGF).
- MSCs grown in medium'>serum-free medium containing DMEM, glutamine and SPN served as untreated controls.
- Due to species-specific toxicity, when inducing mouse MSCs the concentrations of IBMX and cAMP were halved in the step two medium.
Cell Culture
-
H1 (WA01) cells from WiCell were expanded in ES cell growth media and differentiated according to our laboratory’s established protocols
EBs ) were formed by suspending undifferentiated ES cell colonies in ES growth media on ultra low adhesion culture dishes. - The neural differentiation was commenced by growing EBs in medium'>N2B27 medium
NPs ). - NPs were then passaged with 0.05% trypsin/ETA and cultured for 3 weeks in N2B27 media with 20 ng/ml of PGF-AA and 20 ng/ml EGF until transplantation.
- A human fibroblast line (HFF1, from ATCC) from neonate foreskins were cultured in 10% fetal bovine serum as previously described
Cell culture
- Immortalized p19ARF-deficient MIM-1-4 hepatocytes were grown on collagen-coated culture dishes in RPMI 1640 plus 10% fetal calf serum, 40 ng/ml human TGF-α , 30 ng/ml human insulin-like growth factor II , 1.4 nM insulin and antibiotics, as described previously (2 and routinely screened for the absence of mycoplasma.
Primary oligodendrocyte-microglia-co-culture
- Primary OL and microglia cells (MG) were prepared from the cerebral hemispheres of Sprague Dawley rats at P1 to P2 using a shaking method of mixed-glia-cultures 2 for 3 days in a medium'>serum-free medium'>basal-defined medium (BDM: DMEM , 0.1% bovine serum albumin , 50 µg/ml human apo-transferrin , 50 µg/ml insulin , 30 nM sodium selenite , 10 nM D-biotin , 10 nM hydrocortisone , 10 ng/ml human recombinant platelet-derived growth factor , and 10 ng/ml human recombinant basic fibroblast growth factor before MG were added.
- Cultures were kept in a humidified incubator at 37°C and 5% CO2.
- Purified OL contained <5% astrocytes and <1% microglia and purified microglia contained <0.1% astrocytes and <1% oligodendrocytes.
- OL-MG-ratio was set to 1∶1 following cell count results in the rat brain
Differentiation to a Schwann cell phenotype
- Human ADSCs were induced into neurospheres.
- Briefly, we harvested human ADSCs (80–90% confluence) and then plated them in plastic dish a concentration of 1–2 × 105/cm2 in DMEM: F12 supplemented with 20 ng/ mL EGF , 20 ng/mL basic fibroblast growth factor (bFGF) and 2% B27 (1:50) at 37°C in 5%CO2.
- We added fresh medium every 3 to 4 days.
- After 7 days, neurospheres were triturated using a fire-polished Pasteur pipette and re-plated in Laminin coated six-well chamber slides contain DMEM: F12 supplemented with 10% FBS, 14 μM forskolin , 5 ng/mL platelet-derived growth factor-AA (PDGF, ), 10 ng/mL bFGF and 200 ng/mL recombinant human heregulin-beta1 (HRG) for terminal differentiation.
- The cells were incubated for 9 days under these conditions, and then harvested for investigation.
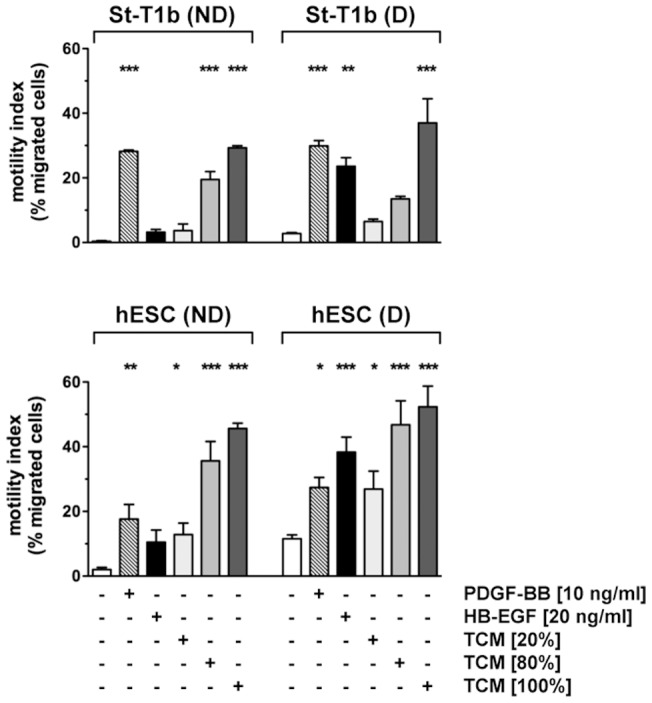
Chemotactic response of St-T1b cells or primary hESCs to PDGF-BB, HB-EGF and trophoblast conditioned medium.
- The bottom reservoir contained MM1-10% (controls), PDGF-BB, HB-EGF or conditioned medium from the trophoblast cell line AC-1M88 undiluted (100%) or diluted to 80% or 20%.
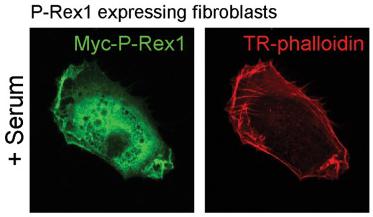
P-Rex1 requires a factor present in serum in order to drive alterations in cell shape and invasion; PDGF can substitute for serum to drive invasion of P-Rex1 expressing cells.
- The invasive migration of vector control (pLHCX) or P-Rex1 expressing fibroblasts into Matrigel in the absence or presence of a serum (0–10%) or PDGF-BB (0–50 ng ml−1) gradient was assayed and quantified as for Figure 3B (±SEM, ***P<0.0005, Mann-Whitney rank sum test, 3 independent experiments).
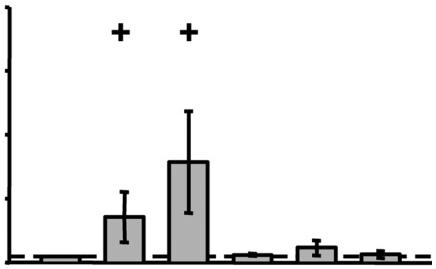
Influence of selected cytokines and growth factors on the IL-33 synthesis in RA-SFs.
- RA-SFs (n=4) were stimulated for 24 h with TNF-α, IL-1β, IL-18, PDGF-BB or TGF-1β (concentrations as indicated in the figure).
Influence of selected cytokines and growth factors on the IL-33 synthesis in RA-SFs.
- RA-SFs (n=4) were stimulated for 24 h with TNF-α, IL-1β, IL-18, PDGF-BB or TGF-1β (concentrations as indicated in the figure).
Isolation and culture of glioma cells
- Glioma biopsies of fresh tumor tissue were obtained from patients operated at the Clinic of Neurosurgery, Lund University Hospital, Sweden.
- Written informed consent was obtained from patients.
- For preparing viable glioma cells, the fresh specimen were cut into small pieces, and incubated in IMDM with 0.5 mg/ml collagenase and 25 mg/ml DNAse at 37°C for 40 minutes.
- Red cells were lysed with NH4Cl.
- The remaining glioma cells were washed in PBS containing 2% fetal calf serum (FCS).
- Glioma cells were grown in poly-L-Lysine (PLL) coated flasks with medium'>MEM/F12 (1∶1) medium supplemented with 2% FCS, --Glucose solution (0.6%), heparin (5 µg/ml), sodium bicarbonate (0.1%), N2 supplement , FGF2 (20 ng/ml), sonic hedgehog (SHH) (2 ng/ml of C24II version& ), and PGF-AA(20 ng/ml).