Human Thrombopoietin Recombinant
Categories: Recombinant Human CytokinesSingle chain family$70.00 – $3,900.00
Description
Accession
P40225
Source
Optimized DNA sequence encoding HumanTPO erythropoeitin domain was expressed in Chinese Hamster Ovary cells.
Molecular weight
Native Human TPO, generated by the proteolytic removal of the signal peptide and propeptide,and has a calculated molecular mass of approximately35 kDa. RecombinantTPO is monomer protein consisting ofamino acid residue subunits, and migrates due to glycosylation as an approximately80 kDa protein under non-reducing conditions and reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent proliferation of Mo7ecells, was found to be in the range of.0-2.0 ng/ml.
Protein Sequence
MELTELLLVV MLLLTARLTL SSPAPPACDL RVLSKLLRDS HVLHSRLSQC PEVHPLPTPV LLPAVDFSLG EWKTQMEETK AQDILGAVTL LLEGVMAARG QLGPTCLSSL LGQLSGQVRL LLGALQSLLG TQLPPQGRTT AHKDPNAIFL SFQHLLRGKV RFLMLVGGST LCVRRAPPTT AVPSRTSLVL TLNELPNRTS GLLETNFTAS ARTTGSGLLK WQQGFRAKIP GLLNQTSRSL DQIPGYLNRI HELLNGTRGL FPGPSRRTLG APDISSGTSD TGSLPPNLQP GYSPSPTHPP TGQYTLFPLP PTLPTPVVQL HPLLPDPSAP TPTPTSPLLN TSYTHSQNLS QEG
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Thrombopoietinwas lyophilized from a 0.2 μm filtered solution inPBS,pH.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
P40238
Molecular function
Molecular function
Methods
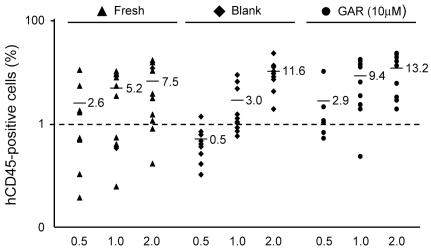
Garcinol efficiently expands SRC numbers.
- A. hCB CD34+ cells were cultured with 10 µM of GAR in the presence of rhSCF, rhTPO and rhFL for 7 days.
RT-PCR using cDNA from human megakaryocytes or cell lines
- Buffy coat peripheral blood (PB) cells were obtained from volunteer blood donors.
- PB was collected after written consent that was obtained from the volunteer blood donors.
- All the protocols were approved by the ethic committees in Japanese Red Cross Osaka Blood Center and Osaka University.
- AC133+ cells were isolated from PB mononuclear cells by a MACS AC133 Cell Isolation Kit , as described previously + cells were cultured in IMDM containing 20% human serum, 50 IU/ml penicillin, 50 µg/ml streptomycin, and 10 ng/ml thrombopoietin (TPO) .
- The same amount of TPO was added to the medium every two days, and half of the medium was replaced with new medium after 6 days of incubation.
- Cells were incubated for 0, 4, 6, or 10 days and then processed for total RNA using ISOGEN ( gene, , ).
- Each cDNA was synthesized from 0.5 to 1 µg of total RNA…
Processing whole blood samples
- Peripheral (PB.1 and PB.2) and cord (CB.1 and CB.2) blood-derived CD34+ cells were obtained from AllCells .
- Blood collections were performed at AllCells and using standard, 8 ml Vacutainer Cell Processing Tubes (both sodium citrate and sodium heparin-based tubes are acceptable ; , ).
- Appropriate documentation for informed consent was completed prior to blood collection .
- Vacutainers were processed within 24 hours of collection.
- Briefly, the PBMC-containing upper phase was collected and washed with ice-cold PBS .
- Cells were either frozen down or used directly for purification with the CD34 MicroBead Kit and used according to the manufacturer's protocol.
- Some samples were treated with Histopaque ( ; St. Louis, ) to minimize the number of red blood cells and centrifuged at 2000 rpm for 20 minutes without braking.
- The interface containing the PBMCs was removed if samples were treated with histopaque, cells washed again with chilled…
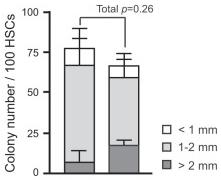
Effects of overexpression of Bmi1 on HSCs in vitro.
- Single CD34-LSK cells were sorted into 96-well microtiter plates containing the SF-O3 medium supplemented with 10% FBS and multiple cytokines (10 ng/ml SCF, 10 ng/ml TPO, 10 ng/ml IL-3, and 3 u/ml EPO) and allowed to form colonies.
Effects of overexpression of Bmi1 on HSCs in vitro.
- Single CD34-LSK cells were sorted into 96-well microtiter plates containing the SF-O3 medium supplemented with 10% FBS and multiple cytokines (10 ng/ml SCF, 10 ng/ml TPO, 10 ng/ml IL-3, and 3 u/ml EPO) and allowed to form colonies.
Cell Culture
- Frozen female CD34+ CBCs were supplied by Bio-Resource Center .
- C34+ CBCs were cultured in hematopoietic culture medium [serum-free X-Vivo10 containing 50 ng/mL IL-6 , 50 ng/mL sIL-6 , 50 ng/mL SCF , ten ng/mL TPO , and 20 ng/mL Flt3/4 ligand ].
- Reprogrammed cells were cultured in feeder-less primate ES cell medium Repro FF (, .
- No. RCHEMD004), ReproFF2 (ReproCELL, cat No. RCHEMD006), mTeSR1 ( catalog number 05850) or E8 (16) supplemented with five ng/mL bFGF (total bFGF ten ng/mL) on Pronectin F-coated dishes.
- Passage of human iPSCs was previously described
Gene Delivery into CD34+ Cells by Retrovirus
- The CD34+ cells isolated from cord blood were cultured in X-VIVO15 , supplemented with 1% human serum albumin (HSA) and stimulated with a cytokine cocktail [100 ng/ml stem cell factor, 100 ng/ml Flt-3 ligand, 100 ng/ml thrombopoietin, and 100 ng/ml IL-6 ] in a 24-well plate (2×105 per well) for 48 h. The stimulated CD34+ cells were then harvested and placed into non-tissue culture-treated 6-well plates that had been coated with 20 µg/ml CH-296, a recombinant fibronectin fragment .
- (3×105 cells per well) in the presence of the respective virus supernatant.
- The virus supernatants were diluted 1∶2 with X-VIVO15 containing 1% HSA and the cytokine cocktail described above.
- Every 12 h, the medium was replaced with fresh virus supernatant.
- After 48 h of culture, the frequency of GFP- and/or Venus-expressing CD34+ cells was examined by FACS.
In vitro Differentiation of miPSCs
- To allow miPSCs to differentiate into medium'>EBs, iPSCs were trypsinized and collected in complete medium'>EB differentiation medium (medium'>EBD) 5 cells per 10 ml medium'>EBD.
- The medium was changed on day four of culture and every two days thereafter.
- On day six, EBs were trypsinized and stained with phycoerythrin-conjugated (PE-) anti-mouse CD41 and allophycocyanin-conjugated (APC-) anti-mouse c-Kit antibodies and sorted CD41+,c-Kit+ cells on OP9 cells.
- OP9 cells were maintained in α-MEM containing 15% FCS.
- 105 OP9 cells were plated in each well of a 6-well tissue culture plate two days before starting co-culture.
- Co-cultures were employed with IMDM containing 20 ng/ml mouse stem cell factor (SCF) and 20 ng/ml human thrombopoietin (TPO) , 10% FCS, 2 mM L-Gln, 0.1 mM 2-ME, and 100 U/ml penicillin/streptomycin.
- On day four of co-culture, cells were recovered…
Induction of 2nd iPSCs
- All isolated somatic cells were cultured in the presence of Dox (2 ug/ml) for induction of 2nd iPSCs.
- Fibroblasts and hematopoietic cells were cultured in ES/iPSC medium.
- FLCD45 were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse EPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6, 10 ng/ml mouse Flt3 ligand, 10 ng/ml mouse GM-CSF, 10 ng/ml mouse VEGF and 50 ng/ml mouse SCF .
- HSCs, HPCs and MPs were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6 and 10 ng/ml mouse Flt3 ligand .
- Macrophages were cultured in the presence of 5 ng/ml M-CSF .
Induction of 2nd iPSCs
- All isolated somatic cells were cultured in the presence of Dox (2 ug/ml) for induction of 2nd iPSCs.
- Fibroblasts and hematopoietic cells were cultured in ES/iPSC medium.
- FLCD45 were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse EPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6, 10 ng/ml mouse Flt3 ligand, 10 ng/ml mouse GM-CSF, 10 ng/ml mouse VEGF and 50 ng/ml mouse SCF .
- HSCs, HPCs and MPs were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6 and 10 ng/ml mouse Flt3 ligand .
- Macrophages were cultured in the presence of 5 ng/ml M-CSF .